To gravimetrically analyze the silver content – Gravimetric analysis of silver content plays a crucial role in determining the purity and concentration of silver in various materials. This technique involves precise measurements and chemical reactions to separate and quantify silver ions, providing valuable insights into the composition and quality of silver samples.
This comprehensive guide delves into the principles, methods, procedures, and applications of gravimetric analysis of silver, empowering readers with a thorough understanding of this essential analytical technique.
1. Gravimetric Analysis of Silver Content
Gravimetric analysis is a technique used to determine the concentration of an analyte in a sample by measuring its mass. In the context of silver analysis, gravimetric methods involve precipitating silver ions from a solution, filtering the precipitate, and weighing it to determine the amount of silver present.
1.1 Principles of Gravimetric Analysis
Gravimetric analysis relies on the principle that the mass of a precipitate is proportional to the amount of analyte in the sample. The precipitate is formed by adding a reagent that reacts with the analyte to form an insoluble compound.
The precipitate is then filtered, washed, dried, and weighed. The mass of the precipitate is used to calculate the concentration of the analyte in the sample.
1.2 Steps Involved in Gravimetric Analysis, To gravimetrically analyze the silver content
- Sample preparation
- Precipitation
- Filtration
- Washing
- Drying
- Weighing
- Calculation
2. Methods for Gravimetric Analysis of Silver
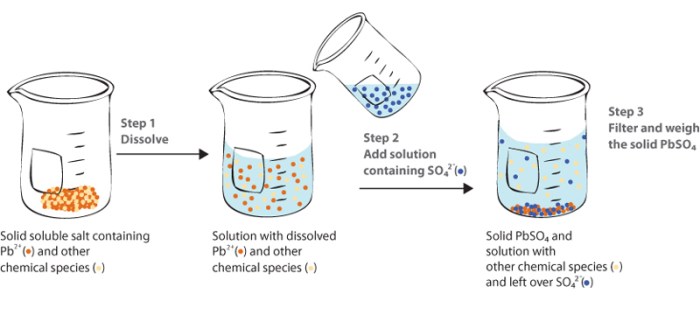
There are several different methods for gravimetric analysis of silver, each with its own advantages and disadvantages. The following table summarizes the most common methods:
| Method | Advantages | Disadvantages |
|---|---|---|
| Precipitation as Silver Chloride | Simple and inexpensive | Requires a large sample size |
| Precipitation as Silver Bromide | More sensitive than silver chloride | More difficult to filter |
| Precipitation as Silver Iodide | Most sensitive method | Requires a large excess of iodide |
The chemical reactions involved in each method are as follows:
- Precipitation as Silver Chloride: Ag+ + Cl- → AgCl
- Precipitation as Silver Bromide: Ag+ + Br- → AgBr
- Precipitation as Silver Iodide: Ag+ + I- → AgI
3. Procedures for Gravimetric Analysis of Silver
The detailed procedures for gravimetric analysis of silver are as follows:
3.1 Sample Preparation
The sample is first dissolved in an appropriate solvent. The solvent should be one that will not react with the silver or the precipitating agent.
3.2 Precipitation
The precipitating agent is added to the sample solution. The precipitating agent is a reagent that will react with the silver ions to form an insoluble precipitate. The precipitate is allowed to form for a period of time, and then it is filtered.
3.3 Filtration
The precipitate is filtered using a filter paper. The filter paper is washed with water to remove any remaining impurities. The precipitate is then dried in an oven.
3.4 Weighing
The dried precipitate is weighed. The mass of the precipitate is used to calculate the concentration of silver in the sample.
3.5 Safety Precautions
Gravimetric analysis of silver can involve the use of hazardous chemicals. It is important to take appropriate safety precautions, such as wearing gloves and eye protection.
3.6 Quality Control Measures
It is important to use quality control measures to ensure the accuracy of the results. These measures may include using a known sample to calibrate the equipment and using a blank sample to check for contamination.
4. Applications of Gravimetric Analysis of Silver: To Gravimetrically Analyze The Silver Content
Gravimetric analysis of silver is used in a variety of applications, including:
- Mining: Gravimetric analysis is used to determine the silver content of ores.
- Jewelry: Gravimetric analysis is used to determine the purity of silver jewelry.
- Environmental monitoring: Gravimetric analysis is used to determine the concentration of silver in environmental samples.
FAQ Insights
What are the advantages of gravimetric analysis?
Gravimetric analysis offers several advantages, including high accuracy and precision, minimal interference from other ions, and the ability to analyze solid samples directly.
What are the limitations of gravimetric analysis?
Gravimetric analysis can be time-consuming and requires careful attention to detail. It is also not suitable for analyzing samples with very low concentrations of silver.
What are the applications of gravimetric analysis of silver content?
Gravimetric analysis of silver content finds applications in various fields, such as determining the purity of silver bullion, analyzing the silver content in jewelry, and monitoring silver levels in environmental samples.